A Theoretical Study on the Compressibility Factor of Hydrogen Gas in the High Pressure Tank
2023 The Korean Hydrogen and New Energy Society. All rights reserved.
Abstract
The fast refueling process of compressed hydrogen has an important impact on the filling efficiency and safety. With the development and use of hydrogen energy, the demand for precision measurement of filling hydrogen thermodynamic parameters is also increasing. In this paper, the compressibility factor calculation model of high-pressure hydrogen gas was studied, and the basic equation of state and thermo-physical parameters were calculated. The hydrogen density data provided by the National Institute of Standards and Technology was compared with the calculation results of each model. Results show that at a pressure of 0.1-100 MPa and a temperature of 233-363 K, the calculation accuracy of the Zheng-Li equation of state was less than 0.5%. In the range of 0.1-70 MPa, the accuracy of Redich-Kwong equation is less than 3%. The hydrogen pressure more influences on the compressibility factor than the hydrogen temperature does. Using the Zheng-Li equation of state to calculate the compressibility factor of on-board high pressure hydrogen can obtain high accuracy.
Keywords:
High pressure hydrogen, Compressibility factor, Equation of state, Thermal physical parameters, Theoretical analysis키워드:
고압수소, 압축성 인자, 상태방정식, 열물리적 매개변수, 이론 해석1. Introduction
With the increasing depletion of fossil energy worldwide and the growing tension in energy supply, it has become a trend to actively explore new energy sources to replace the non-renewable traditional fossil energy sources1). As a clean and efficient new energy source, hydrogen energy is considered to be the most attractive and promising new energy source in the future because of its high combustion calorific value, clean and non-polluting, extensive sources, convenient storage and transportation, and various forms of utilization2,3). With the development and utilization of hydrogen energy, the requirements for accurate metering of high-pressure hydrogen are increasing.
In practice, the flow rate of hydrogen in pipeline systems, the fast filling process at hydrogen filling stations and the hydrogen utilization rate are the main focus of hydrogen applications, which are calculated based on the equation of state for hydrogen. However, under high pressure conditions, the actual hydrogen is completely different from the ideal gas; therefore calculations based on the ideal gas equation of state can cause significant errors4). Mohamed and Paraschivoiu5) used the Beattie-Bridgeman equation of state to study the thermal properties of the hydrogen emission process. Schefer et al6). proposed to use the Abel-Noble equation of state in the hydrogen storage tank nozzle to calculate the properties of the real gas of hydrogen. Later, Khaksarfard et al7). studied the release of high-pressure hydrogen using a numerical method with a real gas model. Xiao et al8). developed an engineering model for predicting the hydrogen leakage distribution when the storage pressure was up to 100 MPa; an adiabatic expansion model was applied to calculate the parameters of the leakage port in their model. Bonelli et al9). investigated the hydrogen fast charging process for filling pressures up to 75MPa; three equations of state were used for the analysis, including ideal gas, Redlich-Kwong (RK) and van der Waals (VDW) equation of state, to investigate the effect of real gas on the jet characteristics. The results show that the real gas can significantly affect the Mach number, pressure and temperature of the jet structure. A systematic study of the outflow coefficients of sonic nozzles with high-pressure hydrogen as the medium was carried out in the literature10), which showed that the compressibility factor and specific heat ratio of hydrogen had a significant effect on the outflow coefficients of sonic nozzles.
Hu et al11). improved the double-layer splitting model of Li et al12). for modelling a high-pressure hydrogen jet blocked by a vertical plate; the Abel-Noble equation of state was chosen to calculate the flow parameters, Mach region and slip region in the storage tank. Zhou et al13). and Yu et al14). calculated the exit conditions of the leakage gas stream based on the Abel-Nobel gas equation of state and compared them with experimental results. Proust et al15). used the Abel-Nobel gas equation of state to predict gas density and pressure changes in storage tanks under different leak hole diameters, and showed that the Abel-Nobel gas equation of state was closer to the experimental values16). Zou et al17). recently reviewed seven real gas equations of state, including Abel-Nobel, VDW, Peng-Robinson (PR), Soave-RK (SRK), RK, Aungier-RK and Beattie Bridgeman, in a comprehensive manner. The Abel-Nobel gas equation of state was chosen to model the high-pressure hydrogen leakage.
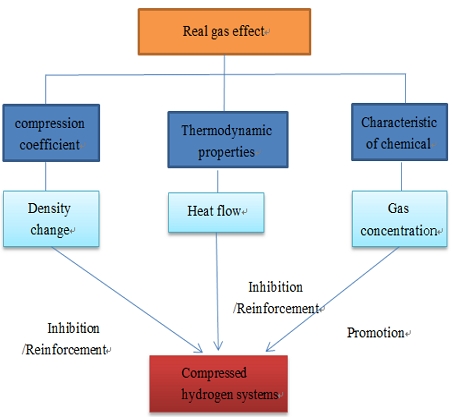
The real gas equation of state is a key part of the hydrogen fast charging process and its role is shown in Fig. 1. Many studies have revealed the need for a real gas equation of state to model the thermal properties of high pressure hydrogen.
The gas equation of state is a function of the density, pressure and temperature of the gas; the gas compressibility factor Z represents the extent to which the actual gas deviates from the ideal gas properties and is an important physical parameter in the measurement of actual hydrogen. Although there are most researches on compressibility factors, there is less research on the calculation of compressibility factors by theoretical methods. This paper investigates models for the calculation of compressibility factors for four commonly used gas equations of state. Finally, a regression analysis with hydrogen density data from the National Institute of Standards and Technology is carried out to compare the results with an accurate model for the calculation of the compressibility factor for high pressure hydrogen.
2. Hydrogen compressibility factor
2.1 Real gas equation of state
At higher gas pressures, higher densities, and lower temperatures, the molecular movement patterns in the gas are complex. The actual conditions of high pressure hydrogen (2-100 MPa) discussed in this study are such conditions. In this case, the equation of state of ideal gas cannot be used to study the p-v-T relationship for the actual state of hydrogen, and the more complex actual gas equation of state is required. In general, the actual gas equation of state is obtained by theoretical and experimental modification of the ideal gas equation of state. A common way of expressing the actual gas equation of state is by introducing a compressibility factor Z=f (p, T) to modify the ideal gas equation of state by a compressibility factor.
2.2 Compressibility factor
The compressibility factor Z represents the deviation in volume of the real gas when it is compressed compared to the ideal gas when it is compressed by the same pressure. The compressibility factor Z is invoked to correct the ideal gas equation of state.
| (1) |
At equation (1), it is clear that the essence of the compressibility factor is the correction factor of the actual gas or gas in its actual state to the ideal gas equation of state (Z=1).
3. Compressibility factor calculation model
In practice, the Joule-Thomson (JT) effect of hydrogen in pipeline systems, the fast filling process at hydrogen filling stations and the utilization rate of hydrogen are the main focus of hydrogen applications, which are calculated based on the equation of state for hydrogen. In the case of hydrogen, the Joule-Thomson effect is not commonly observed because hydrogen molecules have a linear structure, which is already at its lowest possible symmetry. So it is better to explain how JT effect of hydrogen in pipeline systems is significant. However, at high pressure conditions, the actual hydrogen is completely different from the ideal gas; therefore. In order to investigate the compressibility factor of high-pressure hydrogen, the selection of a reasonable and correct equation of state for the real gas is the most essential prerequisite for this study. In this paper, four cubic equations of state were proposed to be used for numerical analysis18-21).
| (2) |
| (3) |
| (4) |
| (5)22) |
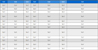
The parameters in the equation of state and the critical parameters for hydrogen are shown in Table 1.
Although there is a wealth of research on compressibility factors, with a large amount of experimental data and empirical formulas, there is relatively little research on calculating compressibility factors by theoretical methods. The general cubic equation of state can be expressed as equation (6).
| (6) |
In this paper, the compressibility factor of the equation of state obtained with PV=ZRT is as follows.
| (7) |
4. Result and discussion
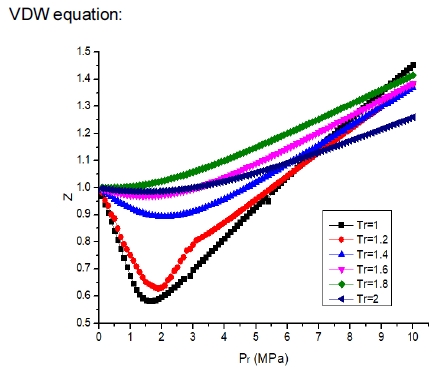
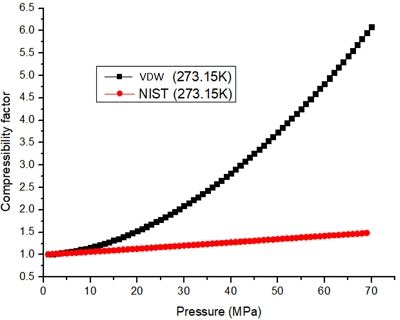
Fig. 2 shows the compressibility factor curve based on the VDW equation. As can be seen from Fig. 3, the hydrogen density increases with increasing pressure, and the error in the VDW equation increases with increasing pressure. Comparing the calculated results with the NIST data, it is found that the error of the VDW equation is larger when P>20 MPa. The pressure of the on-board hydrogen storage cylinder is in the range of 35-70 MPa, so the VDW equation is not applicable to calculate the high-pressure hydrogen compressibility factor.
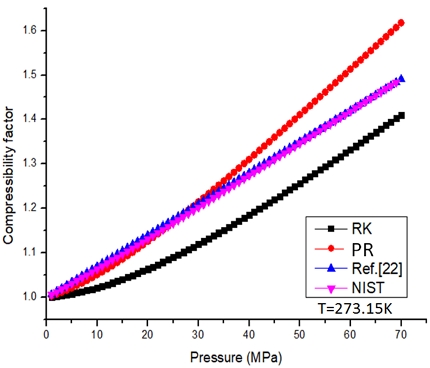
Fig. 4 shows comparison of compressibility factor under different equations of state. The calculated results show that Zheng-Li equation of state22) is in good agreement with the NIST high-pressure hydrogen compressibility factor data. When the pressure is 1-70 MPa, the error is less than 0.5%. However, for high pressure hydrogen with P>35 MPa, the maximum error of compressibility factor calculated by PR equation is less than 5% compared with NIST data. For hydrogen of 0-35 MPa, RK equation has a good calculation accuracy.
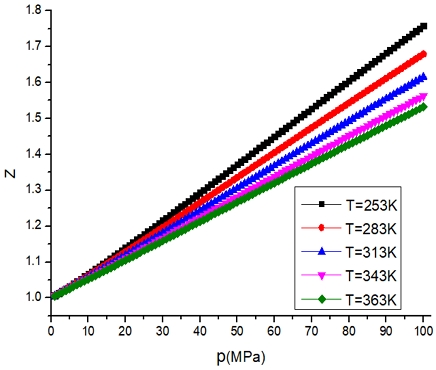
It can be seen from Fig. 5 that the compressibility factor of hydrogen increases with the increase of pressure. The compressibility factor of hydrogen decreases with the increase of temperature under the same pressure. At the same temperature, the compressibility factor increases with the increase of pressure. The hydrogen pressure increases from 0.1 MPa to 100 MPa, and the compressibility factor increases by 70.22%. The temperature increased from 253 to 363 K, and the compressibility factor decreased by 4.89%. The hydrogen pressure more influences on the compressibility factor than the hydrogen temperature does.
5. Conclusion
In response to the lack of accuracy of current methods for calculating the high-pressure hydrogen compressibility factor, an accurate calculation model of high-pressure hydrogen compressibility factor is obtained through comparison. This paper discussed the several calculation models for calculating the high-pressure hydrogen compressibility factor. The conclusions of which are as follows:
1) The compressibility factor of high pressure hydrogen gas is studied by using VDW, RK, PR state equations, and the expression of the compressibility factor is derived.
2) The VDW equation for the compressibility factor of hydrogen is simple and easy to calculate, and is independent of the gas composition. When P<15 MPa, the VDW equation can be used with high accuracy, with a maximum error of <1.8%.
3) In the range of 0.1-100 MPa and temperature 233-363 K, the accuracy of calculation of Zheng-Li equation is <0.5%. For the hydrogen pressure of 0-35 MPa, RK equation has good calculation accuracy. In the range of 0-70 MPa, the accuracy of RK equation is less than 3%. The hydrogen pressure more influences on the compressibility factor than the hydrogen temperature does.
4) NIST data is used to verify the gas equation of state in reference22), and high accuracy can be obtained by using the Zheng-Li equation of state to calculate the high-pressure hydrogen compressibility factor.
5) The temperature range during fast charging of on-board hydrogen storage cylinders was 233.15-358.15 K. The NIST density data was converted to compressibility factor data to explore the trend of compressibility factor with pressure at different temperatures, and the influence of temperature and high pressure on the compressibility factor of compressed hydrogen was obtained.
Nomenclature
| a,b,k,m : | Constants related to the critical parameter of hydrogen gas. |
| p : | The pressure of hydrogen (MPa). |
| T : | The temperature of hydrogen (K). |
| R : | Hydrogen constant 8.31441 (J/[mol • K]). |
| Z : | Hydrogen compressibility factor. |
| pc : | The critical pressure of hydrogen (pc = 1.2966MPa). |
| pr : | The contrast state parameter (). |
| Tr : | The contrast state parameter (). |
| Tc : | The critical temperature of hydrogen (Tc =33.24K). |
| v : | The specific volume of hydrogen. |
| ω : | The acentric factor. |
Acknowledgments
This research was supported by Regional Innovation Strategy (RIS) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-004). This research is also the result of receiving the support for the university innovation support project at Hoseo University, Korea and Yantai next generation industrial robot and intelligent manufacturing engineering laboratory.
References
-
S. J. Oh, J. H. Yoon, K. S. Jeon, and J. J. Choi, “A study on the thermal characteristics of hydrogen storage vessel related to condition of charging”, Journal of Mechanical Science and Technology, Vol. 36, 2022, pp. 1579-1586.
[https://doi.org/10.1007/s12206-022-0243-2]

-
B. H. Park, “Simulation of temperature behavior in hydrogen tank during refueling using cubic equations of state”, Journal of Hydrogen and New Energy, Vol. 30, No. 5, 2019, pp. 385-394.
[https://doi.org/10.7316/KHNES.2019.30.5.385]

-
B. H. Park, “Calculation and comparison of thermodynamic properties of hydrogen using equations of state for compressed hydrogen storage”, Journal of Hydrogen and New Energy, Vol. 31, No. 2, 2020, pp. 184-193.
[https://doi.org/10.7316/KHNES.2020.31.2.184]

-
M. Yang, Y. Jiang, J. Liu, S. Xu, and A. Du, “Lattice Boltzmann method modeling and experimental study on liquid water characteristics in the gas diffusion layer of proton exchange membrane fuel cells”, International Journal of Hydrogen Energy, Vol. 47, No. 18, 2022, pp. 10366-10380.
[https://doi.org/10.1016/j.ijhydene.2022.01.115]

-
K. Mohamed and M. Paraschivoiu, “Real gas simulation of hydrogen release from a high-pressure chamber”, International Journal of Hydrogen Energy, Vol. 30, No. 8, 2005, pp. 903-912.
[https://doi.org/10.1016/j.ijhydene.2004.10.001]

-
R. W. Schefer, W. G. Houf, T. C. Williams, B. Bourne, and J. Colton, “Characterization of high-pressure, underexpanded hydrogen-jet flames”, International Journal of Hydrogen Energy, Vol. 32, No. 12, 2007, pp. 2081-2093.
[https://doi.org/10.1016/j.ijhydene.2006.08.037]

-
R. Khaksarfard, M. R. Kameshki, and M. Paraschivoiu, “Numerical simulation of high pressure release and dispersion of hydrogen into air with real gas model”, Shock Waves, Vol. 20, No. 3, 2010, pp. 205-216.
[https://doi.org/10.1007/s00193-010-0260-4]

-
J. Xiao, J. R. Travis, and W. Breitung, “Hydrogen release from a high pressure gaseous hydrogen reservoir in case of a small-leak”, International Journal of Hydrogen Energy, Vol. 36, No. 3, 2011, pp. 2545-2554.
[https://doi.org/10.1016/j.ijhydene.2010.05.069]

-
F. Bonelli, A. Viggiano, and V. Magi, “A numerical analysis of hydrogen underexpanded jets under real gas assumption”, Journal of Fluids Engineering, Vol. 135, No. 12, 2013, pp. 121101.
[https://doi.org/10.1115/1.4025253]

-
J. Q. Li, J. C. L. Li, K. Park, and J. T. Kwon, “Investigation on the changes of pressure and temperature in high pressure filling of hydrogen storage tank”, Case Studies in Thermal Engineering, Vol. 37, 2020, pp. 102143.
[https://doi.org/10.1016/j.csite.2022.102143]

-
J. Hu, D. M. Christopher, and X. Li, “Simplified partitioning model to simulate high pressure under-expanded jet flows impinging vertical obstacles”, International Journal of Hydrogen Energy, Vol. 43, No. 29, 2018, pp. 13649-13658.
[https://doi.org/10.1016/j.ijhydene.2018.05.036]

-
X. Li, D. M. Christopher, E. S. Hecht, and I. W. Ekoto, “Comparison of two-layer model for hydrogen and helium jets with notional nozzle model predictions and experimental data for pressures up to 35 MPa”, International Journal of Hydrogen Energy, Vol. 42, No. 11, 2017, pp. 7457-7466.
[https://doi.org/10.1016/j.ijhydene.2016.05.214]

-
K. Zhou, J. Liu, Y. Wang, M. Liu, Y. Yu, and J. Jiang, “Prediction of state property, flow parameter and jet flame size during transient releases from hydrogen storage systems”, International Journal of Hydrogen Energy, Vol. 43, No. 27, 2018, pp. 12565-12573.
[https://doi.org/10.1016/j.ijhydene.2018.04.141]

-
X. Yu, Y. Wu, Y. Zhao, and C. Wang, "Flame characteristics of under-expanded, cryogenic hydrogen jet fire", Combustion and Flame, Vol. 244, 2022, pp.112294.
[https://doi.org/10.1016/j.combustflame.2022.112294]

-
C. Proust, D. Jamois, and E. Studer, “High pressure hydrogen fires”, International Journal of Hydrogen Energy, Vol. 36, No. 3, 2011, pp. 2367-2373.
[https://doi.org/10.1016/j.ijhydene.2010.04.055]

-
K. Zhou, X. Wang, M. Liu, and J. Liu, “A theoretical framework for calculating full-scale jet fires induced by high-pressure hydrogen/natural gas transient leakage”, International Journal of Hydrogen Energy, Vol. 43, No. 50, 2018, pp. 22765-22775.
[https://doi.org/10.1016/j.ijhydene.2018.10.122]

-
Q. Zou, Y. Tian, and F. Han, “Prediction of state property during hydrogen leaks from high-pressure hydrogen storage systems”, International Journal of Hydrogen Energy, Vol. 44, No. 39, 2019, pp. 22394-22404.
[https://doi.org/10.1016/j.ijhydene.2019.06.126]

-
R. Laghaei, A. E. Nasrabad, and B. C. Eu, “Generic van der Waals equation of state, modified free volume theory of diffusion, and viscosity of simple liquids”, The Journal of Physical Chemistry B, Vol. 109, No. 12, 2005, pp. 5873-5883.
[https://doi.org/10.1021/jp0448245]

-
O. Redlich and J. N. S. Kwong, “On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions”, Chemical Reviews, Vol. 44, No. 1, 1949, pp. 233-244.
[https://doi.org/10.1021/cr60137a013]

-
G. Soave, “Equilibrium constants from a modified Redlich-Kwong equation of state”, Chemical Engineering Science, Vol. 27, No. 6, 1972, pp. 1197-1203.
[https://doi.org/10.1016/0009-2509(72)80096-4]

-
D. Y. Peng and D. B. Robinson, “A new two-constant equation of state”, Industrial & Engineering Chemistry Fundamentals, Vol. 15, No. 1, 1976, pp. 3069-3078.
[https://doi.org/10.1021/i160057a011]

-
J. Zheng, L. Li, R. Chen, P. Xu, and F. Kai “High pressure steel storage vessels used in hydrogen refueling station”, Journal of pressure vessel technology, Vol. 130, No. 1, 2008, pp. 014503.
[https://doi.org/10.1115/1.2826453]