Synergistic Effect of Sulfonated Poly(Ether Ether Ketone)/Strontium Zirconate Perovskite Nanofiber-Based Novel Electrospun Composite Membranes for Fuel Cell Applications
2022 The Korean Hydrogen and New Energy Society. All rights reserved.
Abstract
In this work, sulfonated poly (ether ether ketone) (SPEEK) composite membranes including strontium zirconate (SrZrO3) were fabricated by the electrospinning method. Fourier-transform infrared spectroscopic analysis and X-ray diffraction analysis were used to identify the chemical structure and the crystallinity of SrZrO3 and electrospun composite membranes. The thermal stability of the pure SPEEK and SPEEK/SrZrO3 electrospun composite membranes were investigated by using thermogravimetric analysis. The physicochemical properties and proton conductivity were enhanced with the addition of different weight ratio of SrZrO3 nanofiller (2, 4 and 6 wt%) in SPEEK polymer.
The optimized SPEEK/SrZrO3-4 electrospun membrane containing 4 wt% of SrZrO3 showed a high proton conductivity compared to other electrospun SPEEK/SrZrO3 composite membranes. The results indicate that electrospun composite membranes incorporating these perovskite nanofillers should be explored as potential candidates for use in proton exchange membrane fuel cells.
Keywords:
Perovskite structure, PEM, Oxidative stability, SrZrO3, Electrospun nanofiber키워드:
패로브스카이트 구조, 양이온 교환막, 산화 안정성, 스트론튬 지르코네이트, 전기방사 나노충전제1. Introduction
Clean energy is a critical issue in modern society. Most of the energy is produced globally from fossil fuels, which have a negative impact on the environment worldwide. To overcome this issue, non-disruptive alternative energy conversion technologies are urgently needed1). As a result, fuel cells are gaining popularity because of their excellent efficiency and nearly zero emissions from fuel and oxidants2). In particular, the proton exchange membrane fuel cells (PEMFCs) provide high-efficiency and eco-friendly energy that uses hydrogen and air as fuels to generate electricity via a chemical reaction and produces water as a by-product.
The main component of the PEMFCs is the proton exchange membrane (PEM), which transports protons between the electrodes and prevents the mixing of H2 and O2 gases3-6). At presently, perfluorinated sulfonic acids such as Nafion are the principle material because of their high ionic conductivity and excellent mechanical stability and durability. However, it has a significant disadvantage such as low proton conductivity at high temperature, difficulty in operation at low relative humidity, and high price7,8).
In order to solve aforementioned problems, intensive efforts are being focused on improving the low-cost aromatic proton conducting polymer membranes that can replace Nafion. Numerous studies show that the incorporation inorganic fillers into polymers can improve the polymer structure, physicochemical properties, thermal stability, permselectivity and mechanical properties, and the pore structure and size distribution of the membranes9,10). Such aromatic proton conducting polymers include sulfonated poly(ether sulfone) (SPES), sulfonated poly(ether ether ketone) (SPEEK), sulfonated poly(1,4-phenylene ether ether sulfone) (SPEES), sulfonated poly(benzimidazole) (SPBI) and sulfonated poly(vinyl alcohol) (SPVA) are intensively used in PEMFC applications11-13).
Currently, organic-inorganic electrospun polymer composite materials are receiving much attention due to their excellent structural, physicochemical, thermal, and electrochemical properties. In particular, SPEEK is a proton conducting polymer in nature that can easily control proton conductivity and thermochemical properties by changing the degree of sulfonation (DS). The SPEEK is an aromatic hydrocarbon polymer and its nanofiber morphology can be predicted to improve chemical stability and high proton conductivity through electrospinning. The electrospun polymer composites systems can improve the properties of PEMFC. It provides improved membrane stability and proton conductivity compared to solvent casting14).
In the current investigation, the incorporation of ABO3 perovskite oxides is structurally strong due to its high temperature proton conductivity compared to normal hygroscopic oxides. Strontium zirconate (SrZrO3) is a perovskite material with a pseudocubic in the Pbnm space group. Strontium zirconate is a mixed metal oxides with different structural characteristics, and due to its proton conductivity at high temperatures, it can be widely applied in the field of electronic ceramics and high melting temperature refractory materials, and in the fields of fuel cells and hydrogen sensors. This material exhibits excellent structural and morphological behavior and large metal-oxygen bonding energy.
According to the Lewis acid-base theory, Sr2+ and Zr4+ act as hard acids that react with the -OH group of water molecule. The pseudocubic perovskite of SrZrO3's has attracted attention due to its excellent proton transport ability and ability to generate a strong oxygen deficient vacancy (0<δ<0.5). The oxygen vacancies mentioned above are very good for attracting water molecules (H2O) that have been changed to their hydroxyl form (OH) and transporting protons along the lattice15,16).
In the literature, Lee et al.17) impregnated SPEEK/silicon dioxide (SiO2) nanofibers in Nafion to create a strong pore-filled membrane, which prevented the matrix from swelling (3.3%) at high temperatures (75°C). Salleh et al.18) reported that SPEEK/Closite 15A electrospun polymer composites provided better proton conductivity (80°C), oxidative stability and better electrochemical performance compared to the pure SPEEK electrospun composite membrane. Raja Pugalenthi et al.19) described a SPEEK/SrFeO3 proton conducting electrospun membranes, which provides proton conduction (95 mS cm-1 at 80°C) and cell performance (342 mW cm-2). These results indicate that the inclusion of SrFeO3 in SPEEK matrices has better properties than the cast SPEEK membranes under hydration conditions. Choi et al.20) synthesized strong SPEEK nanofibers with carbon nanotubes (CNTs) to construct a highly efficient nanophase linked polymer membrane. They have a higher Young’s modulus (615 MPa) than pure SPEEK (557 MPa) and better performance at 80°C (1.1 W cm-2) than pure SPEEK (0.8 W cm-2).
Based on the above inspiration, we prepared SPEEK/SrZrO3 electrospun composite membranes by electrospinning method. The electrospun composite membranes prepared with various wt% SrZrO3 in SPEEK matrix were analyzed in terms of ionic conductivity, water uptake, and ion exchange capacity. The structural and morphological properties of SPEEK/SrZrO3 electrospun composite membranes were determined using Fourier-transform infrared (FT-IR), XRD, 1H NMR and SEM analysis. The obtained electrospun composite membranes exhibited excellent proton conductivity, high thermal stability and chemical stability, suggesting that it is suitable as electrolytes for PEMFC applications.
2. Experimental
2.1 Reagents and chemicals
Poly(ether ether ketone) (PEEK) were obtained from Solvay chemicals PVT, Gujarat, India. Concentrated sulfuric acid (H2SO4, 98%), N,N-dimethylformamide (DMF, 95%), acetone (98%), strontium nitrate (SrNO3, 98%), sodium hydroxide (NaOH, 97%), sodium chloride (NaCl, 99%), zirconium nitrate (ZrNO3, 99%), potassium hydroxide (KOH, 94%), hydrogen peroxide (H2O2, 30% v/v) were purchased from Sigma-Aldrich, Mumbai, India. All experiments were carried out using deionized (DI) water.
2.2 Synthesis of SPEEK
PEEK was sulfonated via a previously reported classical method21). The PEEK powder was dried in a vacuum oven before reaction. 10 g of dried SPEEK was slowly mixed to 150 mL of conc. H2SO4, and then solution was stirred at 40°C for 7 hours and the polymer solution was precipitated in cold water to complete the sulfonation. The yellow precipitate was washed repeatedly with DI water until a neutral pH was reached.
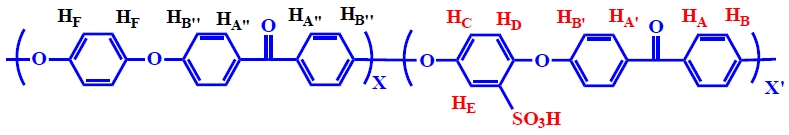
The SPEEK (Fig. 1) with the following 1H NMR data was produced in approximately 90% yield: 1H NMR (600 MHz, DMSO-d6) δ 7.9-7.70 (HA, HA’, HA’’, 8H), 7.6-7.45 (HE, 1H), 7.3-7.2 (HF, HD, 5H), 7.2-7.05 (HB’, HB’’, HC, 7H), 7.05-6.95 (HB, 2H).
2.3 Synthesis of SrZrO3 nanoparticles
1 M strontium nitrate, 1 M zirconium nitrate and 0.5 M potassium hydroxide were mixed together in a 1:1 ratio and stirred for 12 h. The centrifugation process was manipulated to separate the solid particles from the remaining solvent. The obtained powder was calcined by 900°C.
2.4 Preparation of SPEEK/SrZrO3 electrospun composite membranes
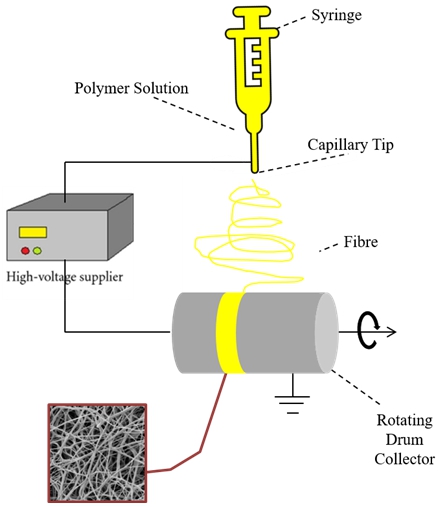
Electrospun membranes containing 0, 2, 4 and 6 wt% of SrZrO3 perovskite filler and named as SPEEK, SPEEK/SrZrO3-2, SPEEK/SrZrO3-4 and SPEEK/SrZrO3-6, respectively. To start, SPEEK was added to 5 mL of DMF with vigorous stirring at 30°C for 24 h. Then, a sufficient amount of SrZrO3 was distributed in the polymer solution using sonication and stirring at 80°C for 24 hours. Using an ectrospinning technique, nanofibers were made from a prepared polymer solution. Electrospinning of the solution was carried out at a voltage of 22 kV and a flow rate of 0.3 mL h-1.
The electrospun membrane was received on a spinning drum at a speed of 1500 revolutions per minute and covered with aluminum foil (at the distance of 11 cm from syringe spout). The electrospun composite membrane was dried at 70°C for 9 hours to reduce any residual solvent. The thicknesses of the prepared electrospun composite membranes were calculated in the range of 30 and 40 μm using a digital micrometer. The aforementioned process is shown in Fig. 2.
Each SPEEK/SrZrO3 electrospun membrane was impregnated with 10 mL of a solution of 5 wt% Nafion in 5 mL isopropanol with 0.5 mL water (3/2, w/w), respectively. Finally, the prepared electrospun membranes were immersed in 0.5 M H2SO4 for the activation process.
2.5 Characterization
FT-IR spectra of the electrospun composite membranes were taken between 4000 and 400 cm-1 on a (FT-IR, Perkin Elmer, Waltham, USA). The phase structure of the electrospun composite membranes was characterized using a powder X-ray diffractometer (PXRD, X'Pert MRD with Cu Kα radiation). 1H NMR was recorded on a Bruker 400 MHz NMR spectrometer.
The morphological behavior of the electrospun composite membranes was investigated using a scanning electron microscope (SEM, SUPRA 40VP). The thermal stability of the electrospun membranes was determined using thermogravimetric analysis (TGA) model (Q 50) heating in an N2 environment at 10°C min-1.
The proton conductivity of the prepared electrospun composite membranes was evaluated from a Nyquist plot obtained from impedance measurements of a computer-controlled micro auto lab type III potentiostat/Galvanostat with a signal amplitude of 10 mV and a frequency range of 10 Hz∼10 MHz. The proton conductivity (σ) was then calculated using the Nyquist plot and the electrolyte resistance (Rb) determined by the intersection of the X-axis, the membrane thickness (L) and the electrode area (A) according to the equation (1):
| (1) |
The water uptake of electrospun composite membranes was measured by the change in weight of the electrospun composite membranes having a size of 1 cm×1 cm, and then immersed in DI water at 30°C for 24 h to maintain the equilibrium state. After elimination of surface water for 24 hours, the electrospun membranes were quickly weighed and the results recorded. The water uptake of the membranes was calculated using equation (2):
| (2) |
The ion exchange capacity(IEC) of the electrospun composite membranes was determined using a back-titration approach. To begin, the acidic membranes (H+) were converted to the Na+ form by immersion in 1 M NaCl solution at 30°C for 24 hours. Then, the H+ ions exchanged in the solution were titrated with 0.01 M NaOH solution using phenolphthalein as an indicator. Finally, the IEC was calculated as in the following equation (3):
| (3) |
In addition, the chemical stability of the prepared composite membranes was evaluated through the Fenton’s test. The electrospun composite membrane obtained here was initially soaked in Fenton’s regent (3 ppm of FeSO4 with 3% H2O2 solution) at 80°C for 134 hours. The resultant membrane was then dried at 80°C, and the initial and final weight loss was calculated.
3. Results and Discussion
3.1 1H NMR study
The 1H NMR spectra of the prepared SPEEK was measured using DMSO-d6 solvent. These proton signals are in good agreement with those reported previously22). The DS of SPEEK was 65%.
3.2 FT-IR study
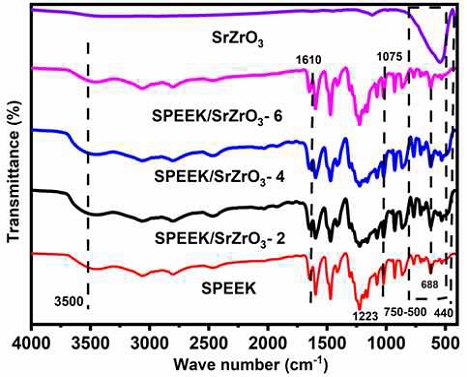
Fig. 3 shows the FT-IR spectrum of pristine SPEEK, SrZrO3 and electrospun membranes SPEEK/SrZrO3-2, SPEEK/SrZrO3-4 and SPEEK/SrZrO3-6. In the vibrational spectrum of SPEEK there was a broad peak in the region 3500 cm-1 confirming the OH stretch of SO3H group of SPEEK. In the spectrum of SrZrO3, two vibrational peaks were observed at 440 cm-1 and 533 cm-1, which correspond to the presence of Zr-O and Sr-O stretching vibration modes in SrZrO3, respectively16).
FT-IR analysis of the SPEEK/SrZrO3 electrospun membranes shows that the intensity of bending vibration of the hydroxyl group at 1610 cm-1 and 3,500 cm-1 increases with increasing filler content in SrZrO3 due to the additional hydroxyl groups present in the filler23). The observed vibrational peaks at 400∼750 cm-1 of the prepared electrospun composite membranes indicate the metal-oxygen stretching vibrations at the B site of ABO3 perovskite24). The above results confirm that the SPEEK/SrZrO3 composite is formed by hydrogen bonding between the SPEEK sulfonic acid group and the SrZrO3 functional group.
3.3 XRD analysis
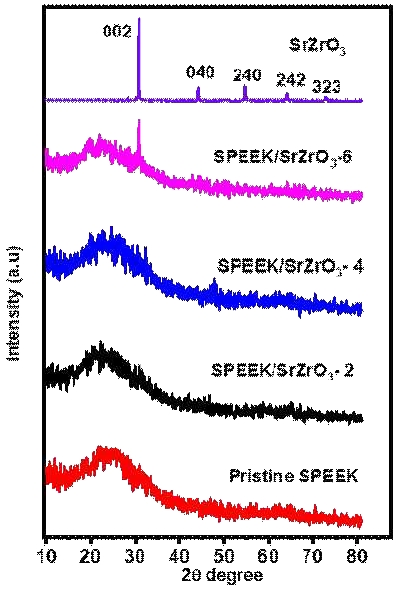
XRD analysis is a powerful tool for determining the structural behavior. Fig. 4 shows the XRD patterns of pristine SPEEK, pristine SrZrO3 and prepared electrospun composite membranes. In Fig. 4, the pure SPEEK membrane exhibited a wide centered peak at 2θ=20°, confirming the semi-crystalline structure. It confirms the loss of crystallinity and chain conformation25). The pure SrZrO3 exhibits five major peaks at 2θ=30.85º, 44.13º, 54.66º, 63.89º and 72.54º represents the crystal planes of (002), (040), (240), (242), and (323)16). The average particle size was calculated by using the Scherrer’s equation as follows.
| (4) |
- λ = X-ray wavelength, β = full-width half maximum, θ = diffraction angle
The average particle size of the SrZrO3 nanoparticles was found to be 55 nm. The SPEEK membrane and SPEEK/SrZrO3 electrospun composite membranes exhibit a broad diffraction peak between 20∼30º indicating the amorphous nature. The peak intensity at 6 wt% SrZrO3 increases in the prepared electrospun membranes due to the agglomeration effect of SrZrO3 occurring in the electrospun membrane.
3.4 Water uptake, ion exchange capacity and oxidative stability
Water up take is one of the important properties for fuel cell system operation. Increased water uptake by electrospun membrane can promote better proton transport26-28). The water uptake of electrospun composite membranes generally increases with increasing IEC properties due to their increased hydrophilic nature. The SrZrO3 fillers increased the water retention capacity of SPEEK membranesr. The water uptake was performed at 30°C and the obtained values were calculated according to equations (2) and (3), and the corresponding calculation results are shown in Table 1. As a result, the water uptake values for all the manufactured electrospun membranes were greater than those of pure SPEEK electrospun membranes. Table 1 shows that the prepared SPEEK/SrZrO3-4 electrospun membrane demonstrated the highest water uptake value of 17.34%, which is more reasonable for the utillization of the operating electrospun membranes in fuel cells under humid conditions.
In addition, IEC is one of the major properties of proton conducting polymers, which can be improved by adding ion exchange fillers. The pure SPEEK electrospun membrane exhibited an IEC of 1.2 meq g-1 as a result of contribution by sulfonic acid group (SO3H). Increasing the amount of SrZrO3 in the SPEEK electrospun membrane increased the ion exchange capacity as shown in Table 1. A maximum IEC of 2.5 meq g-1 was shown by the SPEEK/SrZrO3-4 electrospun membrane. Whereas, the SPEEK/SrZrO3-6 electrospun membrane showed lower IEC values due to the agglomeration of SrZrO3 particles, which blocks the ion exchange groups from participating the ion exchange process.
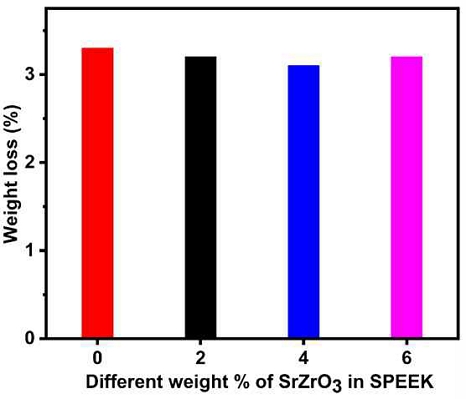
The chemical stability of prepared electrospun composite membranes as estimated using Fenton's reagent method is one of the essential prerequisites for the fuel cell operation. The pure SPEEK electrospun membrane and the prepared electrospun composite membrane showed a weight loss of less than 6 wt% after immersion in Fenton's reagent for 134 hours (Fig. 5). This shows that the electrospun membranes have good chemical stability. Disruption of the ether-bonded SPEEK polymer chains can cause a decrease in the stability of the electrospun membrane.
3.5 Thermal stability
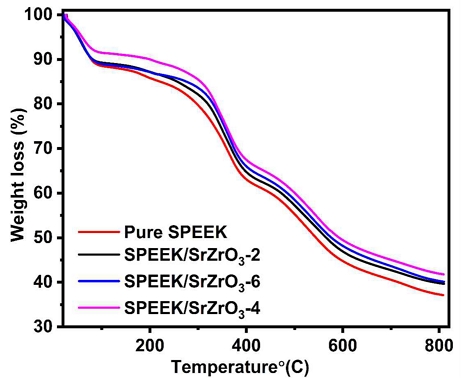
The thermal stability of the prepared electrospun membranes was investigated using TGA analysis at heating rate of 10 °C min-1 in an N2 atmosphere. The TGA curve of prepared electrospun membranes is shown in Fig. 6. Pure SPEEK and prepared SPEEK/SrZrO3 electrospun membranes show the first loss corresponding to moisture on sample loading29). The second weight loss occurring at 250∼400°C is caused by the degradation of the sulfonic acid group (SO3H) in the prepared electrospun membranes30). The rapid third weight loss occurring at 400∼800°C proceeds with the decomposition of aromatic chains of sulfonated polymers bound to the pure SPEEK electrospun membrane, and the prepared electrospun membrane exhibits delayed behavior of the weight droplets31,32).
The TGA curves of electrospun composite membranes showed a slightly delayed weight loss compared to the pure SPEEK due to the electrostatic interactions between the SrZrO3 and -SO3H groups of SPEEK electrospun membrane. The obtained results demonstrate that TGA shows high thermal properties of the SPEEK/SrZrO3-4 electrospun composite membrane for PEMFC applications.
3.6 Morphology study
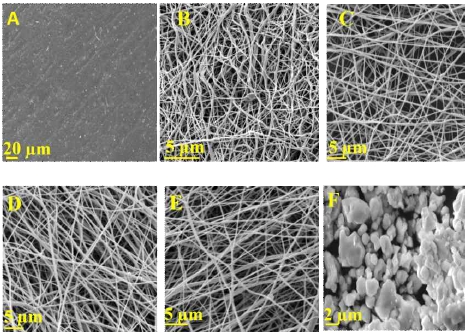
The surface morphologies of the pristine SPEEK (casted and electrospun), pure SrZrO3 and prepared electrospun membranes are represented in Fig. 7. Fig. 7A shows the smooth and uniform surface morphology of a pore-free SPEEK(cast) membrane. The SEM images of SPEEK and SPEEK/SrZrO3 electrospun membranes are shown in Fig. 7B∼E. The filler particles are dispersed in a homogeneous pattern (fibers) and are clearly embedded in the SPEEK polymer matrix33). The SrZrO3 particles are well dispersed in the SPEEK matrix without aggregation in the prepared electrospun because there is a strong electrostatic attraction with sulfonic acids (SO3H) between SrZrO3 and SPEEK. The results obtained were similar to those of previous works34,35).
3.7 AC impedance
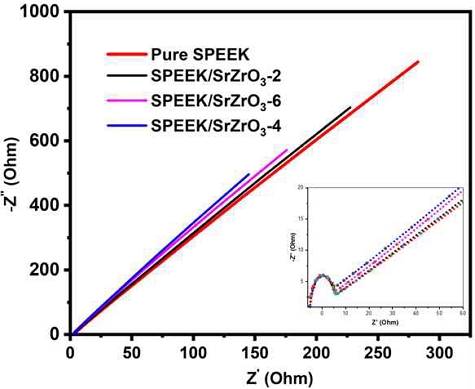
The Nyquist plot of SPEEK, SPEEK/SrZrO3-2, SPEEK/SrZrO3-4 and SPEEK/SrZrO3-6 at 30°C are shown in Fig. 8. According to Fig. 8, the real part of the x-axis is denoted by Z' and the imaginary part is denoted by -Z". The bulk resistance Rb can be calculated from the intercept on the x axis. The proton conductivity was calculated from the bulk resistance of the electrospun membranes of Fig. 8 using equation (1). The SPEEK electrospun membrane achieved a conductivity of 6.43×10-2 S cm-1 at 30°C. Compared to this membrane, the SPEEK/SrZrO3-4 electrospun membrane increased the conductivity up to 13.62×10-2 S cm-1 at 30°C due to highly conductive electrospun composite membrane. Increasing the amount of filler particle in the SPEEK electrospun membrane increased the proton conductivity as shown in Table 1.
The proton conductivity of electrospun membrane is enhanced by two possible factors, such as the excessive concentration of ion-conducting SO3H provided by the high surface area of SrZrO3 nanoparticles and the intrinsic ability of SrZrO3 nanoparticles to retain their physical and chemical properties36-39). In SrZrO3, proton transport is facilitated by hydrogen bonding between adjacent lattice oxygen atoms and reorientation of related oxygen sites. The oxygen site of Zr-O-Zr lowers the energy barrier for ion transport and large cation at the A-site contributes to a reduced activation enthalpy for ion mobility40,41).
The incorporation of the SrZrO3 increases the water adsorption properties of SPEEK electrospun membrane, and the adsorbed water molecules increase the size of ion cluster domains and the strong interconnection and dissociation of acidic functional groups. For membranes with 6 wt% SrZrO3 filler, a slight decrease in proton conductivity was observed due to particle agglomeration disrupting the ionic channel of the electrospun membrane.
4. Conclusions
The present study described aspects of SrZrO3 perovskite in SPEEK electrospun membranes as electrolytes for PEMFC applications. The synthesized SrZrO3 and the prepared electrospun membranes were characterized by NMR, XRD, FTIR, TGA, and SEM to investigate their physicochemical properties.
In the presence of SrZrO3, the SPEEK electrospun membranes showed better physicochemical properties and proton conductivity compared to the pristine SPEEK. The electrospun with 4 wt% of SrZrO3 showed the best results compared to other electrospun composite membranes. The highest proton conductivity was obtained at 13.62×10-2 S cm-1 at 30°C for SPEEK/SrZrO3-4 electrospun composite membrane. The aggregation and blocking effect of SrZrO3 at 6 wt% of the SrZrO3 filler reduced the proton conductivity. Therefore, the SPEEK/SrZrO3-4 based electrospun composite membrane is a promising electrolyte for PEMFC applications.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2020R1A2B5B01001458). This research was supported by Basic Science Research through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01050905).
References
-
H. Wang, X. Liang, J. Wang, S. Jiao, and D. Xue, “Multifunctional inorganic nanomaterials for energy applications”, Nanoscale, Vol. 12, No. 1, 2020, pp. 14-42.
[https://doi.org/10.1039/C9NR07008G]

-
V. Elumalai, C. K. Kavya Sravanthi, and D. Sangeetha, “Synthesis characterization and performance evaluation of tungstic acid functionalized SBA-15/SPEEK composite membrane for proton exchange membrane fuel cell”, Appl. Nanosci., Vol. 9, 2019, pp. 1163-1172.
[https://doi.org/10.1007/s13204-019-01005-5]

-
D. J. Yoo, S. H. Hyun, A. R. Kim, G. G. Kumar, and K. S. Nahm, “Novel sulfonated poly(arylene biphenylsulfone ether) copolymers containing bisphenylsulfonyl biphenyl moiety: structural, thermal, electrochemical and morphological characteristics, Polym. Int., Vol. 60, No. 1, 2010, pp. 85-92.
[https://doi.org/10.1002/pi.2914]

-
J. Y. Chu, A. R. Kim, K. S. Nahm, H. K. Lee, and D. J. Yoo, “Synthesis and characterization of partially fluorinated sulfonated poly(arylene biphenylsulfone ketone) block copolymers containing 6F-BPA and perfluorobiphenylene units”, Int. J. Hydrog. Energy, Vol. 38, No. 14, 2013, pp. 6268-6274.
[https://doi.org/10.1016/j.ijhydene.2012.11.144]

-
K. H. Lee, J. Y. Chu, A. R. Kim, and D. J. Yoo, “Effect of functionalized SiO2 toward proton conductivity of composite membranes for PEMFC application”, Int. J. Energy Res., Vol. 43, No. 10, 2019, pp. 5333-5345.
[https://doi.org/10.1002/er.4610]

-
A. R. Kim, M. Vinothkannan, S. Ramakrishnan, B. H. Park, M. K. Han, and D. J. Yoo, “Enhanced electrochemical performance and long-term durability of composite membranes through a binary interface with sulfonated unzipped graphite nanofibers for polymer electrolyte fuel cells operating under low relative humidity”, Appl. Surf. Sci., Vol. 593, 2022, pp. 153407.
[https://doi.org/10.1016/j.apsusc.2022.153407]

-
G. Rambabu, N. Nagaraju, and S. D. Bhat, “Functionalized fullerene embedded in Nafion matrix: a modified composite membrane electrolyte for direct methanol fuel cells”, Chem. Eng. J., Vol. 306, 2016, pp. 43-52.
[https://doi.org/10.1016/j.cej.2016.07.032]

-
U. Thanganathan, S. Kumar, A. Kishimoto, and K. Kimura, “Synthesis of organic/inorganic hybrid composite membranes and their structural and conductivity properties”, Mater. Lett., Vol. 72, 2012, pp. 81-87.
[https://doi.org/10.1016/j.matlet.2011.12.066]

-
U. Thanganathan, “Structural study on inorganic/organic hybrid composite membranes”, J. Mater. Chem., Vol. 21, No. 2, 2011, pp. 456-465.
[https://doi.org/10.1039/C0JM02504F]

-
K. Divya, D. Rana, M. S. Sri Abirami Saraswathi, and A. Nagendran, “Sulfonated poly(ether sulfone) composite membranes customized with polydopamine coated molybdenum disulfide nanosheets for renewable energy devices”, Polymer, Vol. 175, 2019, pp. 255-264.
[https://doi.org/10.1016/j.polymer.2019.05.001]

-
S. Neelakandan, P. Kanagaraj, R. M. Sabarathinam, A. Muthumeenal, and A. Nagendran, “SPEES/PEI-based highly selective polymer electrolyte membranes for DMFC application”, J. Solid State Electrochem., Vol. 19, 2015, pp. 1755-1764.
[https://doi.org/10.1007/s10008-015-2784-0]

-
W. Qian, Y. Shang, M. Fang, S. Wang, X. Xie, J. Wang, W. Wang, J. Du, Y. Wang, and Z. Mao, “Sulfonated polybenzimidazole/zirconium phosphate composite membranes for high temperature applications”, Int. J. Hydrog. Energy, Vol. 37, No. 17, 2012, pp. 12919-12924.
[https://doi.org/10.1016/j.ijhydene.2012.05.076]

-
J. A. Quinn, Y. Yang, A. N. Buffington, F. N. Romero, and M. D. Green, “Preparation and characterization of crosslinked electrospun poly(vinyl alcohol) nanofibrous membranes”, Polymer, Vol. 134, 2018, pp. 275-281.
[https://doi.org/10.1016/j.polymer.2017.11.023]

-
M. Raja Pugalenthi, M. Ramesh Prabhu, “The pore filled SPEEK nanofibers matrix combined with ethylene diamine modified SrFeO3 nanoneedles for the cation exchange membrane fuel cells”, J. Taiwan Inst. Chem. Eng., Vol. 122, 2021, pp. 136-147.
[https://doi.org/10.1016/j.jtice.2021.04.054]

-
A. Unemoto, A. Kaimai, K. Sato, N. Kitamura, K. Yashiro, H. Matsumoto, J. Mizusaki, K. Amezawa, and T. Kawada, “High-temperature protonic conduction in LaFeO3 - SrFeO3 - δ - SrZrO3 solid solutions”, J. Electrochem. Soc., Vol. 158, No. 2, 2011, pp. 180-190.
[https://doi.org/10.1149/1.3518426]

-
K. Ahmad, P. Kumar, and S. M. Mobin, “A highly sensitive and selective hydroquinone sensor based on a newly designed N-rGO/SrZrO3 composite”, Nanoscale Adv., Vol. 2, No. 1, 2020, pp. 502-511.
[https://doi.org/10.1039/C9NA00573K]

-
C. Lee, S. M. Jo, J. Choi, K. Y. Baek, Y. B. Truong, I. L. Kyratzis, and Y. G. Shul, “SiO2/sulfonated poly ether ether ketone (SPEEK) composite nanofiber mat supported proton exchange membranes for fuel cells”, J. Mater. Sci., Vol. 48, 2013, pp. 3665-3671.
[https://doi.org/10.1007/s10853-013-7162-7]

-
M. T. Salleh, J. Jaafar, M. A. Mohamed, M. N. A. M. Norddin, A. F. Ismail, M. H. D. Othman, M. A. Rahman, N. Yusof, F. Aziz, and W. N. W. Salleh, “Stability of SPEEK/Cloisite®/TAP nanocomposite membrane under Fenton reagent condition for direct methanol fuel cell application”, Polym. Degrad. Stab., Vol. 137, 2017, pp. 83-99.
[https://doi.org/10.1016/j.polymdegradstab.2016.12.011]

-
M. Raja Pugalenthi, C. Liu, G. Cao, and M. Ramesh Prabhu, “Tailoring SPEEK/SPVdF-co-HFP/La2Zr2O7 ternary composite membrane for cation exchange membrane fuel cells”, Ind. Eng. Chem. Res., Vol. 59, No. 11, 2020, pp. 4881-4894.
[https://doi.org/10.1021/acs.iecr.9b06922]

-
J. Choi, C. Lee, S. C. Hawkins, C. P. Huynh, J. Park, Y. Jeon, Y. B. Truong, I. L. Kyratzis, Y. G. Shul, and R. A. Caruso, “Direct spun aligned carbon nanotube web-reinforced proton exchange membranes for fuel cells”, RSC Adv., Vol. 4, No. 62, 2014, pp. 32787-32790.
[https://doi.org/10.1039/C4RA03117B]

-
A. R. Kim, J. C. Gabunada, and D. J. Yoo, “Amelioration in physicochemical properties and single cell performance of sulfonated poly(ether ether ketone) block copolymer composite membrane using sulfonated carbon nanotubes for intermediate humidity fuel cells”, Int. J. Energy Res., Vol. 43, No. 7, 2019, pp. 2974-2989.
[https://doi.org/10.1002/er.4494]

-
K. Selvakumar, S. Rajendran, and M. Ramesh Prabhu, “Influence of barium zirconate on SPEEK-based polymer electrolytes for PEM fuel cell applications”, Ion., Vol. 15, 2018, pp. 2243-2253.
[https://doi.org/10.1007/s11581-018-2613-4]

-
A. M. Huerta-Flores, L. M. Torres-Martínez, D. Sánchez-Martínez, and M. E. Zarazúa-Morín, “SrZrO3 powders: alternative synthesis, characterization and application as photocatalysts for hydrogen evolution from water splitting”, Fuel, Vol. 158, 2015, pp. 66-71.
[https://doi.org/10.1016/j.fuel.2015.05.014]

-
W. Münch, K. D. Kreuer, G. Seifert, and J. Maier, “Proton diffusion in perovskites: comparison between BaCeO3, BaZrO3, SrTiO3, and CaTiO3 using quantum molecular dynamics”, Solid State Ion., Vol. 136-137, 2000, pp. 183-189.
[https://doi.org/10.1016/S0167-2738(00)00304-0]

-
A. R. Kim, M. Vinothkannan, D. J. Yoo, “Sulfonated-fluorinated copolymer blending membranes containing SPEEK for use as the electrolyte in polymer electrolyte fuel cells (PEFC)”, Int. J. Hydrog. Energy, Vol. 42, No. 7, 2017, pp. 4349-4365.
[https://doi.org/10.1016/j.ijhydene.2016.11.161]

- A. R. Kim, “Preparation and characterization of hybrid membrane for block copolymer containing diphenyl unit increasing cationic conductivity for fuel cells”, Trans Korean Hydrogen New Energy Soc, Vol. 28, No. 5, 2017, pp. 465-470.
-
B. H. Oh, A. R. Kim, and D. J. Yoo, “Profile of extended chemical stability and mechanical integrity and high hydroxide ion conductivity of poly(ether imide) based membranes for anion exchange membrane fuel cells”, Int. J. Hydrogen Energy, Vol. 44, No. 8, 2019, pp. 4281-4292.
[https://doi.org/10.1016/j.ijhydene.2018.12.177]

-
H. Ilbeygi, A. F. Ismail, A. Mayahi, M. M. Nasef, J. Jaafar, and E. Jalalvandi, “Transport properties and direct methanol fuel cell performance of sulfonated poly (ether ether ketone)/Cloisite/triaminopyrimidine nanocomposite polymer electrolyte membrane at moderate temperature”, Sep. Purif. Technol., Vol. 118, 2013, pp. 567-575.
[https://doi.org/10.1016/j.seppur.2013.07.044]

-
A. R. Kim, M. Vinothkannan, K. H. Lee, J. Y. Chu, B.-H. Park, M. K. Han, D. J. Yoo, “Enhanced performance and durability of composite membranes containing anatase titanium oxide for fuel cells operating under low relative humidity”, Int. J. Energy Res., Vol. 46, No. 4, 2022, pp. 4835-4851.
[https://doi.org/10.1002/er.7477]

-
N. Awang, M. A. M. Yajid, and J. Jaafar, “Impact of exfoliated structure on the performance of electrospun SPEEK/cloisite nanocomposite membranes as proton exchange membranes for direct methanol fuel cell application”, J. Environ. Chem. Eng., Vol. 9, No. 4, 2021, pp. 105319.
[https://doi.org/10.1016/j.jece.2021.105319]

-
G. Liu, W. C. Tsen, S. C. Jang, F. Hu, F. Zhong, H. Liu, G. Wang, S. Wen, G. Zheng, and C. Gong, “Mechanically robust and highly methanol-resistant sulfonated poly(ether ether ketone)/poly(vinylidene fluoride) nanofiber composite membranes for direct methanol fuel cells”, J. Membr. Sci., Vol. 591, 2019, pp. 117321.
[https://doi.org/10.1016/j.memsci.2019.117321]

-
M. Raja Pugalenthi, R. Gayathri, C. Guozhong, and M. Ramesh Prabhu, “Study of amine customized exfoliated BN sheets in SPEEK-PES based blend membrane for acid-base cation exchange membrane fuel cells”, J. Environ. Chem. Eng., Vol. 10, No. 1, 2022, pp. 107025.
[https://doi.org/10.1016/j.jece.2021.107025]

-
Y. He, H. Zhang, Y. Li, J. Wang, L. Ma, W. Zhang, and J. Liu, “Synergistic proton transfer through nanofibrous composite membranes by suitably combining proton carriers from the nanofiber mat and pore-filling matrix”, J. Mater. Chem. A, Vol. 3, No. 43, 2015, pp. 21832-21841.
[https://doi.org/10.1039/C5TA03601A]

-
P. Li, J. Dang, W. Wu, J. Lin, Z. Zhou, J. Zhang, and J. Wang, “Nanofiber composite membrane using quantum dot hybridized SPEEK nanofiber for efficient through-plane proton conduction”, J. Membr. Sci., Vol. 609, 2020, pp. 118198.
[https://doi.org/10.1016/j.memsci.2020.118198]

- A. R. Kim, “Preparation and characterization of block copolymer containing bisphenyl propane unit and nanosilica composite membrane for fuel cell electrolyte application”, Trans Korean Hydrogen New Energy Soc, Vol. 28, No. 2, 2017, pp 144-149.
-
M. Saiful Islam, R. Andrew Davies, and Julian D. Gale, “Proton migration and defect interactions in the CaZrO3 orthorhombic perovskite: a quantum mechanical study”, Chem. Mater., Vol. 13, No. 6, 2001, pp. 2049-2055.
[https://doi.org/10.1021/cm010005a]

-
T. Hibino, K. Mizutani, T. Yajima, and H. Iwahara, “Characterization of proton in Y-doped SrZrO3 polycrystal by IR spectroscopy”, Solid State Ion., Vol. 58, No. 1-2, 1992, pp. 85-88.
[https://doi.org/10.1016/0167-2738(92)90014-G]

-
T. Yajima, H. Suzuki, T. Yogo, and H. Iwahara, “Protonic conduction in SrZrO3-based oxides”, Solid State Ion., Vol. 51, No. 1-2, 1992, pp. 101-107.
[https://doi.org/10.1016/0167-2738(92)90351-O]

-
K. D. Kreuer, “Proton-conducting oxides”, Annu. Rev. Mater. Res., Vol. 33, 2003, pp. 333-359.
[https://doi.org/10.1146/annurev.matsci.33.022802.091825]

-
L. Welston, A. Janotti, X. Y. Cui, C. Stamp, and C. G. Van de Walle, “Acceptor doping in the proton conductor SrZrO3”, Phys. Chem. Chem. Phys., Vol. 19, No. 18, 2017, pp. 11485-11491.
[https://doi.org/10.1039/C7CP01471F]

-
M. Raja Pugalenthi, G. Cao, and M. Ramesh Prabhu, “Cross-linked SPEEK-PEG-APTEOS-modified CaTiO3 perovskites for efficient acid-base cation-exchange membrane fuel cell”, Energy Fuels, Vol. 34, No. 8, 2020, pp. 10087-10099.
[https://doi.org/10.1021/acs.energyfuels.0c01933]